
Dr Mojgan Najafzadeh, Prof Diana Anderson, Dr Mohammad Isreb
Our dynamic and highly accomplished Cell and Molecular Biology research group studies molecular mechanisms important for cellular function in health and disease with a special emphasis on chronic diseases such as obesity, type II diabetes, cardiovascular disease, cancer and infertility.

Our dynamic and highly accomplished Cell and Molecular Biology research group studies molecular mechanisms important for cellular function in health and disease with a special emphasis on chronic diseases such as obesity, type II diabetes, cardiovascular disease, cancer and infertility.
We investigate molecular mechanisms contributing to atherosclerosis (leading to heart attacks) and restenosis after heart bypass surgery in the ageing population and as a complication of diabetes.
We study inflammatory signaling pathways, angiogenesis, apoptosis and epigenetic modulators (e.g. non coding RNA), on functional responses of endothelial cells, vascular smooth muscle cells and platelets.
We are also developing multicellular models useful in tissue engineering.

Blood pressure and heart rate monitoring
Cancer is a complex disease with many contributing factors.
Whilst it is clear that genetics plays a key role in determining predisposition to developing cancer, environmental factors also play a role and more importantly are modifiable risk factors.
For the cells lining the colon their environment is determined by the foods we eat. There is convincing evidence to show that foods containing dietary fibre decrease the risk of developing colon cancer, whilst red meat and alcoholic drinks increase this risk.
Research within the diet and cancer group is focused on understanding the molecular basis of these effects.
In particular, we are interested in how food products in the colon interact with the gut microbiota to bring about changes in the normal life cycle of the colon epithelial cells.

Cancer is a complex disease with many contributing factors. Whilst it is clear that genetics plays a key role in determining predisposition to developing cancer, environmental factors also play a role and more importantly are modifiable risk factors.
For the cells lining the colon their environment is determined by the foods we eat. There is convincing evidence to show that foods containing dietary fibre decrease the risk of developing colon cancer, whilst red meat and alcoholic drinks increase this risk.
Research within the diet and cancer group is focused on understanding the molecular basis of these effects. In particular, we are interested in how food products in the colon interact with the gut microbiota to bring about changes in the normal life cycle of the colon epithelial cells.
There is considerable interest in genetic defects and disorders of male reproduction, and our work addresses such issues.
We can distinguish heritable genetic damage occurring preconceptionally from that induced during pregnancy and were the first to demonstrate that offspring could inherit damaged DNA from their smoking fathers. Thus, public health advice is now that hopeful Dads should not smoke for 3 months before conception.
There is a good correlation between DNA damage in sperm and lymphocytes, and we have devised a blood test to predict the chance of developing cancer based on DNA damage levels in these white blood cells.
We helped prove that sperm deliver not only DNA-encoded information to the egg at fertilisation but also epigenetic signals (heritable information not contained in the DNA). We are now investigating epigenetic damage in reproductive diseases.
Using bioinformatics, we discovered a protein expressed in the testes only when germ cells are dying and are studying its significance in infertility.
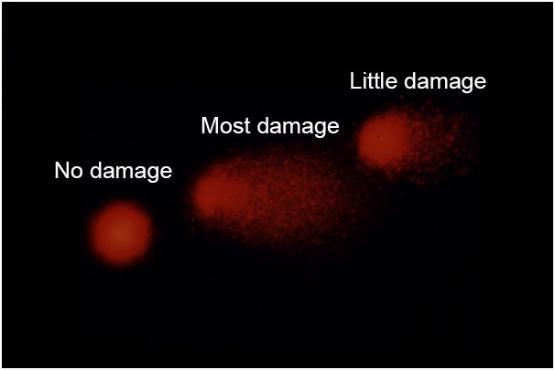
DNA damage in blood lymphocytes in the comet assay
In the neuroendocrinology research theme we are investigating molecular mechanisms in body weight regulation.
The hypothalamus is the brain centre that is important in the regulation of body weight and food intake and whose dysfunction is associated with metabolic disorders such as type 2 diabetes and obesity.
We are interested in how the hypothalamus regulates long-term changes in energy balance and growth and integrates external environmental signals, such as the light/dark cycle or diet, to influence body weight and appetite.
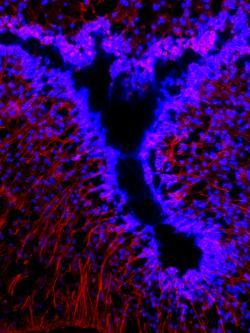
Specialised glial cells, tanycytes (in red), in the hypothalamus are important in body weight regulation

Dr Mojgan Najafzadeh, Prof Diana Anderson, Dr Mohammad Isreb
Dr Lijun Shang, Prof Diana Anderson, Dr Martin Brinkworth

Dr Kirsten Suman-Riches, Dr Julie Thornton
Prof. S. Rimmer, Dr W. Martin

Head of School of Chemistry & Biosciences, Professor of Biochemistry

Professor of Biomedical Science and Established Chair

Associate Professor in Biomedical Sciences

Associate Professor in Medical Sciences

Associate Professor in Biochemistry

Associate Professor in Biomedical Sciences

Associate Professor in Biomedical Science

Assistant Professor in Biomedical Science

Assistant Professor in Medical Research